SECTION C
ANSWER ALL QUESTIONS IN THIS SECTION
9. In order to determine the concentration of a dilute sulphuric acid solution, a student is provided with the following solutions and equipment: 0.25M NaOH, dilute HCl, Pipette, Burette, Conical flask, phenolphthalein indicator, clamp, and stand.
(a) Draw the experimental setup you would use.
( b) 25 cm3 of 0.25M NaOH was transferred into a conical Bask and 3 drops of phenolphthalein indicator was added.
(i) Sketch and name the apparatus used to transfer the NaOH solution
(ii) Identify the liquid that is used to rinse the conical flask
(3 marks)
(c) The dilute acid was run from the burette and two accurate results were recorded as shown on the following diagrams:
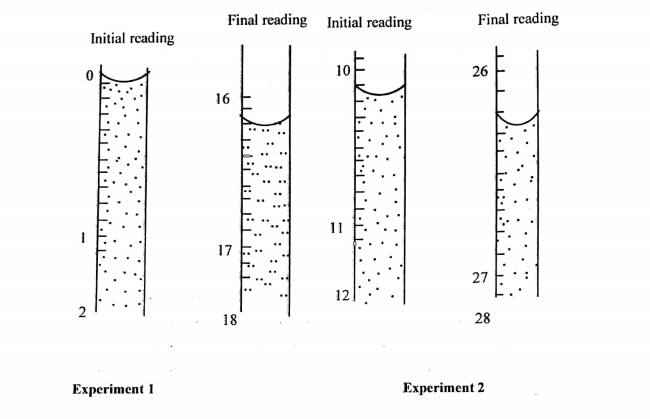
(i) Read and record the results of experiment 1 and 2 on the following table
| Experiment 1 | Experiment 2 | |
| final reading | ||
| initial reading | ||
| Titre |
(3 marks)
(ii) Determine the mean type of dilute sulphuric acid used. (1 mark)
(iii) What will be the colour change to indicate the end of the experiment? ( 1 mark)
(iv) Calculate the concentration of the dilute sulphuric solution if 25cm3 of 0.25M NaOH was required. (2 marks)
(v) In carrying out this experiment the acid accidentally spilled on the student’s hands. Advice the student on what should be done.
(d) You are given the following gases: H2, S02, NH3
Sketch the apparatus you would use to collect a pure sample of each gas
(6 marks)
( Total = 20 marks)
10. In order to determine the chemical composition of three unknown substances X, Y, and Z, a student carried the following lest.
Study the following table and complete the information needed.
| No | TEST PROCEDURE | OBSERVATION | INFERENCE | |
| (i) | To 2cm3 of solution X is added 5 drops of N OH(aq) warmed | A colourless pungent gas is evolved. The gas turns damp red litmus paper blue |
2 | |
| (ii) | To 2cm3 of solution X is added 2 drops of HCl(aq) | Presence of SO2- ions |
2 | |
| (iii) | Presence of Fe2+ ions | 1 | ||
| (iv) | To 2cm3 of solution Y is added a few drops of AgNO3 followed by dil HNO3 |
A white ppt is formed | 1 | |
| (v) | Z gives a brick red flame colour | 3 | ||
| (vi) | A solid sample of Z strongly heated in a test tube |
1 | ||
|
NO2 evolved Presence of NO3– |
10 marks |
(b) Give the chemical identity of
X _________
Y _________
Z __________
(c) Complete the following table to show how you will separate thee mixtures.
| Mixture | Techniques of separation |
| Kerosine and water | |
| Simple distillation | |
| Paper chromatography | |
| Groundnut and its peelings |
(4 marks)
(d) (i) In order to dilute an acid, a student added 410cm3 of distilled water to 10cm3 of concentrated sulphuric acid. State and justify what is wrong with this procedure.
(ii) Why is it advisable to wear eye goggles when working in the laboratory?


