This short lesson notes on radioactivity isn’t a full course but a summary touching the important points. This lesson note is made to assist the student in the class of lower sixth and upper sixth. Ordinary level students can also read this to learn one or two things.
Unstable atomic nuclei will spontaneously release energy in the form of radiation to form a more stable one. This process which is termed Radioactivity is a very useful phenomenon and since it involves the release of huge amounts of energy, it is an exergonic process.
Radioactivity – Definition
Radioactivity is the spontaneous (random) emission of radiation from the nuclei of unstable atoms to form more stable nuclei.
Because the nucleus experiences the intense conflict between the two strongest forces in nature, it should not be surprising that there are many nuclear isotopes which are unstable and emit some kind of radiation.
There are three different type of radiation that can be emitted during a radioactive decay;
- Alpha particles (α-particles)
- Beta particles ( β-particles)
- And gamma rays (γ)
In both α and β emissions, the parent nucleus undergoes a change of atomic number and therefore becomes the nucleus of a different element. This new nucleus is called the daughter nucleus or decay product.
α-decay
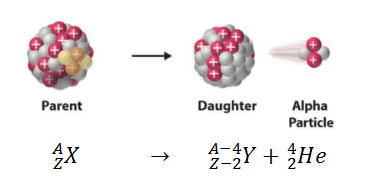
This type of radioactivity occurs when the parent nucleus losses 4 nucleons (a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines an isotope’s mass number), 2 of which are protons.
So, if the parent nucleus is to loss four nucleons, it will inmply that its atomic mass or mass number decreases by 4.
On the other hand, its atomic number will decrease by 2 since the atomic number is defined by the numbers of protons in the nucleus.
So, if our parent nucleus is X with mass number A and atomic or proton number Z, after an α-decay we will have:
β-decay
In this type of radioactive emission, a β-particle is emitted by the unstable parent nucleus resulting in an increase of atomic number while the mass number remains unchanged. That is;
If the parent nucleus is X and has as mass number A and proton number Z, then after a β-decay, we will have;
A good example of a β-decay is exhibited by the Carbon-14 (C-14) Iostope where it emits a β-particle to form N-14 (Nitrogen)
Gamma decay
Gamma decay is the emission of electromagnetic radiation of an extremely high frequency i.e. very high energy, giving out excess energy in order to stabilize the unstable nucleus. You must be quite familiar with the various energy levels in an atom. The Nucleus has its own energy levels. Gamma decay is the nucleus’s way of dropping from a higher energy level to a lower energy level through the emission of high energy photons. The energy level transition energies in the atom are in the order of MeV. Therefore, the gamma-ray emitted is also of very high energy of the order of MeV, just like x-rays. The gamma rays emitted can be differentiated from x-rays only by the fact that gamma rays come from the nucleus. Due to their high energy, they are extremely penetrating and thereby dangerous to biological life forms.
Here we will speak a little further about the distinction between an x-ray and a gamma-ray. X-rays are emitted by electrons (either in the orbits or in outside applications like particle accelerators, synchrotrons radiation, etc) whereas gamma rays are emitted by the nucleus, particle decay, or annihilation reactions.
Unlike, alpha decay and beta decay, the parent nucleus does not undergo any physical change in the process, daughter and parent nuclei are the same. Most of the time, gamma decay occurs after the radioactive nuclei have undergone an alpha or a beta decay. The alpha and beta decays leave the daughter nuclei in an excited state. From the excited state, the daughter nuclei can get back to the ground state by emitting one or more high energy gamma rays.
Sources of Gamma Rays
Sources of gamma rays other than radioactive decay include terrestrial thunderstorms and lightning, from celestial bodies such as pulsars, quasars, distant galaxies, gamma-ray bursts in space and collapse of a star into a black hole known as a hypernova aka super-luminous supernova. Hypernovae events result in bursts of long-duration gamma-ray emissions. These emissions produce a total energy output of about 1044 Joules (as much energy as our Sun will produce in an entire lifetime) in a span of 20-40 seconds.
Gamma rays cause damage on a cellular level and due to their penetrating nature, they can diffuse this damage through the entire body. However, gamma rays are less ionizing that alpha or beta hence the severity is lesser but penetration is more.
Applications of Gamma Rays
Some of the most energetic phenomena in the universe occurs through gamma rays. We cannot witness these events without a gamma-ray detector. To address this, scientists have created a satellite called Fermi Gamma-ray Space Telescope that provides an unparalleled view of the universe. Gamma-ray sensors are also used in the food packaging and chemical industry to measure density, thickness, and composition. Gamma rays are used to treat certain types of cancer where the high energy gamma beams are irradiated on the cancerous cells to kill them.
What next:
- The theory of radioactive decay
- Nuclei stability
- Importance or uses of radioactivity