31. Determine the percentage by mass of nitrogen in NH4N03.(RMM of NH4N03 = 80)
A: 17.5
B: 70
C: 35
D: 22.5
32. Which of these halogens is the most reactive?
A: Chlorine
B: Bromine
C: Iodine
D: Fluorine
33. Which of the following occurs during the electrolysis of aqueous copper (II) sulphate using copper electrodes?
A: The cathode increases in size
B: The anode increase in size
C: The colour of the solution fades
D: Oxygen is discharged at the anode
34. The compound most likely to be present in a bag of fertilizer is:
A: Ca(N03)2
B: (NH4)3P04
C: NH4CI
D: NaCI
Questions 35 – 37
Instructions: Each question consists of a statement in the left-hand column followed by another in the right-hand column. Decide whether each of the statements is true or false. Then on your answer sheet, choose
A: If both statements are TRUE and the second statement is the CORRECT EXPLANATION of the first
B: If both statements are TRUE but the second statement is NOT the correct explanation of the first,
C: If the first statement is true but the second statement is FALSE
D: If the first statement is FALSE but the second statement is TRUE.
| FIRST STATEMENT | SECOND STATEMENT | |
| 35 | Elements of Group VII show similar chemical properties | Group VII elements have same number of shells |
| 36 | The presence of Cl– ,Br– and I ions in solution can be tested using AgN03 solution |
All three ions form coloured precipitates with AgN03 solution |
| 37 | Copper will displace magnesium from a solution of a magnesium salt | Copper is below magnesium in the electrochemical series |
38. Palm wine goes sour when exposed to air for some days because;
A: Glucose is converted to gluconic acid
B: Ethanol is converted to Ethanoic acid
C: Glucose is converted to ethanol
D: Palm wine is converted to palmitic acid
39. Calculate the mass of zinc that will produce 16g of copper according to the equation: Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
A: 1.625
B: 15.75
C: 16.25
D: 1.58
40. Which of the following is a basic oxide?
A: AI2O3
B: S02
C: Na20
D: CO
41. What is the mass of 0.1 mole of Na2CO3? (Rmm of Na2CO3= 106)
A: 106g
B: 10.Og
C: 1.06g
D: 10.6g
42. Plastics pollute the environment because they are;
A: Kerosene and water
B: Ethanol and water
C: NH4CI and NaCl
D: NaCl and H20
43. The different components of universal indicator solution can best be separated by
A: Fractional distillation
B: Filtration
C: Chromatography
D: Simple distillation
44. Consider the equation NaOH(aq) + HCI(aq) → NaCl(aq) + H2O(l) ΔH = X KJ
What does X represent?
A: Heat of neutralization
B: Activation energy
C: Heat of formation
D: Heat of solution
45. 0.1MHC1 reacts with powdered Na2CO3 according to the equation
2HCl(aq) + Na2CO3(s) 2NaCl(s) → 2NaCl(aq) + H20(l) + C02(g). How can the reaction be made to go faster?
A: Using 0.5MHC1
B: Using lumps ofNa2COj
C: Adding water to the reaction mixture
D: Using 0.01MHCI
46. How many particles of NaOH are contained in 0.1 mole of NaOH?
A: 6.02 x 1023
B: 6.02 x 1024
C: 6.02 x 1025
D: 6.02 x 1022
Questions 47 and 48 are based on the diagram below:
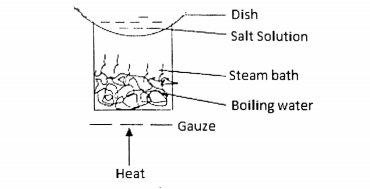
47. What method of separation is illustrated in the diagram?
A: Crystallization
B: Sublimation
C: Evaporation to dryness
D: Filtration
48. An example of a mixture that can be separated by this method is
A: Kerosene and water
B: Ethanol and water
C: NH4CI and NaCl
D: NaCl and H20
49. Select the process that involves a physical change.
A: Heating candle wax
B: Electrolyzing dil. NaCl
C: Burning a piece of Na
D: Corrosion
50. Isotopes of a given element have the same
A: Mass number
B: Number of neutrons
C: Physical properties
D: Atomic number
What next?
Advanced Level students should refer here for Chemistry GCE past papers.



